Our previous post discussed the gradual process in which scientists discovered a link between infants’ health, their gut microbiome, and breast milk. In this post, we’re going to dive deeper into this relationship and understand how the human body has evolved to cultivate thousands of strains of beneficial bacteria in a symbiotic relationship.
By the time Escherich and Tissier conducted their pediatrics and microbiology research on infants’ gut microbiome, chemists were also starting to take an interest in breast milk and its interesting properties. Chemists were the first to note that human milk differs significantly compared to milk produced by other mammals (like cows). A German scientist named Eschbach (not to be confused with Escherich from the previous post!) noted that human milk had a “different” type of lactose than the lactose found in cow’s milk, claiming that those are two different molecules[1]
This finding, however, was soon corrected by a French scientist named George Deniges, who noted[2] that the lactose in human and cow milk are identical. Still, human milk contains, in addition, an unknown carbohydrate fraction.
It took more than 40 years for the next breakthrough to take place. In the early 1930s, two researchers named Polonowski and Lespagnol were able to characterize the “unknown” carbohydrate fraction[3] and gave it the name “gynolactose” (Gyno- comes from the Greek gynḗ, meaning “woman”. Therefore, gynolactose means woman-derived lactose). It had some peculiar chemical properties that were different than lactose and made it easily distinguishable from other types of sugars. primarily, it contained nitrogen — an element that isn’t found in pure lactose or glucose. A few years later, Polonowski and another one of his students (Jean Montreuil) finally separated the “unknown fraction” that was named gynolactose to its pure constituents. However, the exact structure or function of the oligosaccharides they were able to isolate was unknown[4].
It was Richard Kuhn, a German biochemist, who won the Nobel prize back in 1938 for his work on vitamins — who first understood the purpose of those peculiar oligosaccharides by showing their effect on pure samples of gut bacteria. First, he and his team showed that the whey fraction (the liquid fraction of milk after it was curdled and strained, a process that takes place during cheese production) of human milk contains a growth-promoting factor for the anaerobic bacteria called Bifidobacterium Bifidus discovered by Tissier more than 50 years ago[5], (mentioned in our previous post). He called it “the Bifidus factor”, having studied vitamins most of his academic life, he hypothesized that this is a “bacterial equivalent form a vitamin”. Then, intrigued by those findings, they tested the whey fraction of cow’s milk to see whether it has the same growth promotion capabilities on Bifidobacterium Bifidus and found that it had only a negligible growth-promoting capability.
Finally, they were able to explain an observation made at the dawn of the century — Why the Bifidobacterium Bifidus bacteria have only a minor presence in bottle-fed infants? Because bottle-fed infants didn’t get this growth-promoting molecule that was so important for their proliferation! The next step would be discovering what is that elusive molecule. Kuhn and his team eventually confirmed[6] that the Bifidus factor is actually an oligosaccharide (reminder: a few simple sugars linked together) that was part of the gynolactose fraction and was barely present in cow’s milk.
Once it was understood that human milk has a wide range of different oligosaccharides, many research groups worldwide started to characterize them one by one. Interestingly, all those oligosaccharides were built from just a handful of simple sugars that can be assembled in different ways to create all the known repertoire of Human Milk Oligosaccharides (HMO) (See Figure below)
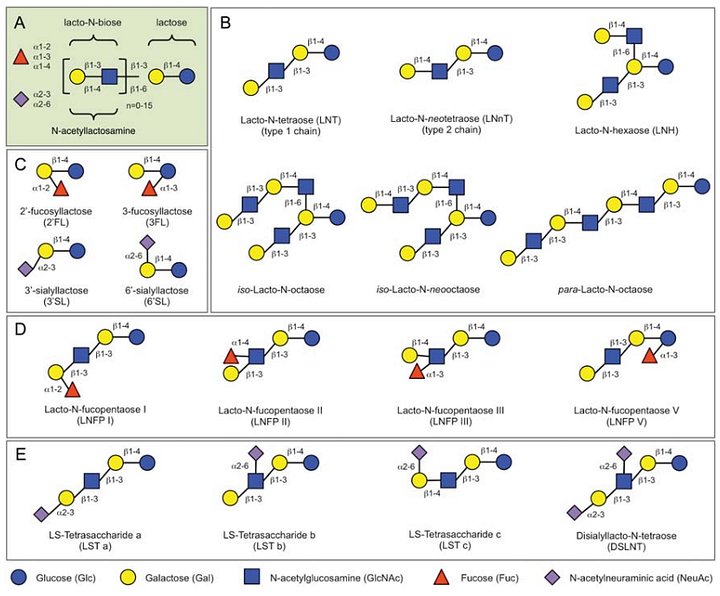
Fucose is one intriguing example (the red-colored triangle). It is a monosaccharide that serves many purposes in mammals, one of those is the determination of blood type[8]. Most blood type systems (there are dozens of ways to classify blood types, the ABO system is just one of them!) use the type of oligosaccharide present on the cell membrane of red blood cells (and/or on other cells) to classify blood types. It turns out that the enzymes used to attach fucose molecules to HMOs (in a chemical process called fucosylation) also play a role in fucosylating the oligosaccharides located on red blood cells[9] (or secreted out of some types of cells). So the repertoire of one’s breast milk HMOscorrelatesd with its blood type (in particular, this is true for the Lewis [9] blood group determinants, which is a system of blood type classification that considers different motifs than the ABO system).
Until now, we discussed the properties of HMOs and traced the series of discoveries that unraveled those properties. In our next post, we’ll discuss the purpose of HMOs and how they influence the immune system and brain development.
1. Montreuil J, Renne B, Sawatzki G. New Perspectives in Infant Nutrition. 1993.
2. Kunz C. Historical aspects of human milk oligosaccharides. Adv Nutr. 2012;3: 430S–9S.
3. Polonowski M, Lespagnol A. Sur la nature glucidique de la substance lévogyre du lait de femme. Bull Soc Biol. 1929;101: 61–63.
4. Polonovski M, Montreuil J. [Chromatographic study of the polyosides of human milk]. C R Hebd Seances Acad Sci. 1954;238: 2263–2264.
5. Schönfeld H. Über die Beziehungen der einzelnen Bestandteile der Frauenmilch zur Bifidusflora. Jahrbuch der Kinderh. 1926;113: 19–60.
6. Gyorgy P, Norris RF, Rose CS. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys. 1954;48: 193–201.
7. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22: 1147–1162.
8. Schneider M, Al-Shareffi E, Haltiwanger RS. Biological functions of fucose in mammals. Glycobiology. 2017;27: 601–618.
9. Blank D, Dotz V, Geyer R, Kunz C. Human milk oligosaccharides and Lewis blood group: individual high-throughput sample profiling to enhance conclusions from functional studies. Adv Nutr. 2012;3: 440S–9S.

