Life is one of the very few concepts in science that lacks a clear definition. Most scientists agree on a set of properties that describe living things, but those don’t define life. One popular definition claims that “organisms are open systems that maintain homeostasis, are composed of cells, have a life cycle, undergo metabolism, can grow, adapt to their environment, respond to stimuli, reproduce and evolve”
Although this definition encompasses all life forms that we are familiar with (viruses/viroids not included) we still can’t rule out that somewhere in the universe, life takes different shapes and forms.
For example (Spoiler Alert!), the classic novel Solaris written by the polish author Stanislav Lem chronicles the communication attempts with an extra-terrestrial life inhabiting a planet called Solaris. This entity is actually a planet-encompassing ocean, which is capable of responding to stimulus and interacting with its surroundings.
The ability to utilize energy to do work, although not unique to living organisms ( your iRobot is just one such example). Is a property shared by all living organisms — from the simplest bacteria to blue whales and most certainly to all life forms in the universe.
That’s because life cannot exist without energy. Energy is the only way we can combat the relentless advance of entropy, the growing disorder in the universe.
In a sense, life is actually a process that involves transforming energy from one form to another.
In this post, I will discuss the fundamental role energy plays in all living organisms and the role enzymes play in harnessing different forms of energy making life as we know it possible.
Energy is what makes life possible.
Imagine you are about to build a cabin in the woods. You put a lot of effort into the design and construction to make sure your cabin will last for generations. For example, you make sure the logs are coated with wax to prevent rot, you build a roof that can withstand the weight of snow in the winter, and so on.
How long would this cabin last without any maintenance? A few years, perhaps. By then, the coating on the logs would start to peel off., fungi would colonize the exposed logs causing them to rot and eventually collapse.
To keep the cabin standing, you need to do work to maintain it. For example, apply more coating when necessary, replace damaged logs, and so on.
Work is a process of converting energy from one form to another. In our example, lifting a wooden log requires your muscle cells to utilize chemical energy stored in them to generate mechanical movement. This movement transfers the kinetic energy generated by your arm to potential energy stored in the wooden log that is lifted.
In the context of biology, those “maintenance” processes enable a state that is another hallmark of life — homeostasis, Homeo — meaning similar or same, and Stasis — meaning state.
Homeostasis is a state created by living cells to maintain an environment hospitable to the plethora of chemical processes occurring within it. An environment in a state of homeostasis is markedly different from its exterior and is resistant to changes going on in the exterior environment. To conclude, cells that are not in homeostasis are dead or about to die.
Similar to the above wooden cabin example, maintaining homeostasis requires work, and therefore energy. For example, our bodies maintain a constant range of temperatures of 36.6–37.0 degrees celsius whether we live in the north pole or the Sahara desert. If the outside temperature rises, our body uses energy to cool down by excreting sweat. If the temperature of our body goes down, our body generates heat by shivering.
The primary source of energy for living organisms is our Sun. The nuclear fusion that takes place in the sun’s core emits energy in the form of photons that are fired to the expanses of space. Only 1 billionth (10–9) of this energy reaches earth.
Plants and other photosynthetic organisms are the only organisms that can directly harness the sun’s energy to perform work. How do they do it? How do plants transfer energy from photons that were emitted millions of kilometers away by the nuclear fusion that occurs in the sun to us?
Humans, as well as many other organisms, harness their energy from food which is usually composed of organic compounds such as sugars and amino acids. Since humans (as well as all non-photosynthetic organisms) can’t directly harness the sun’s energy, we rely on plants to do it for us and consume the molecules they produce.
Indeed — the energy generated by the nuclear fusion in the sun’s core is stored in the banana you ate for breakfast. Specifically, this energy is stored in the chemical bonds that bind carbon, oxygen, and hydrogen together to form those sugars and other more complex molecules.
Let’s run another thought experiment:
Imagine you have a lot of different machines — machines that perform relatively basic tasks — like generating light and heat (or cold) up to very complex machines that take the outputs of simpler machines to create complex outputs. For example — to create cement, you’ll need machines that mine raw materials, machines that use heat for the production process, and so on. The cement produced can then be used as input to machines that construct a wide range of cement-based products like houses and roads.
Almost all of those machines require energy, but they can only work with one energy source and nothing else. Let’s imagine it to be a spring held compressed by a small pin.
The compressed spring in our example functions as a universal energy currency, akin to the electricity coming out from electric sockets.
In such a world, the only type of machines that can compress those springs are analogous to our photosynthetic organisms, and glucose and other types of sugars and starches are the springs. Those molecules store the energy coming from the sun in a very similar fashion to the way mechanically compressed springs store energy.
Let’s define a new concept — the amount of energy stored that is available to do work. Let’s call it G.
The amount of energy available to do work in our example of a compressed spring is higher than the amount of energy available to do work in a non-compressed spring that is in a steady state. The same goes for sugar molecules. The energy stored in the chemical bonds that bind oxygen and carbon together is waiting to be released.
So the change in the amount of energy available to do work (G) between the final state and the starting state is negative! (Gend — Gstart < 0)
What does it mean?
It means that our system has some potential energy that is available. But it doesn’t mean that it will be released spontaneously, at least at a rate that is biologically meaningful.
In our spring analogy, the spring is held in a compressed state by a pin. To release the pin and release the energy, one has to spend a minor amount of energy — perhaps a little shake will do.
Once this tiny amount of energy is spent, a much higher amount of energy is released by the decompression of the spring, ending in a net positive energy balance (that can power other processes and even be used to release the next pin).
Sugar molecules are held together by a “chemical pin” as well. That’s why sugar isn’t oxidized spontaneously.
And that’s also where enzymes come into play. Enzymes are machines that lower the activation energy for breaking chemical bonds and releasing the imaginary pin. If you have ever used a lever to lift something up, you are very much familiar with the concept of “lowering the activation energy.”
Levers convert a small force applied over a long distance to a large force applied over a small distance. Work is the force times the distance, W = Fd, so the total work done is the same with or without the lever. Look closely at a lever as you use it. The end that lifts the payload moves a very small distance as the job is carried out, but the end of the lever that your hand is pushing on moves a large distance as the job is carried out. Because the total work done must be constant, and work is force times distance, the force must go up if the distance goes down. The lever converts the little force of your hand at one end to a large force at the other end; large enough to do a big job. But it does this at the cost of a larger distance. Therefore, you must push on the one end of the lever for a longer time than you would have without the lever, thus lowering the “activation energy” for lifting a payload by dividing it into smaller “chunks” that can be achieved much more easily.
Figure 1: The mechanism of a lever
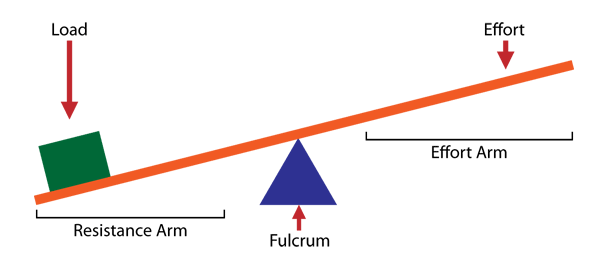
Credit: Koltepranita, CC BY-SA 3.0 via Wikimedia Commons
The lever analogy is a nice example that’s still in the realms of classical mechanics. At the molecular level, things get much more complicated. The driving force of enzyme catalysis is a topic of long-lasting debate among scientists. The predominant theories range from Fischer’s “lock and key” model 1 to Pauling’s and Koshland’s induced fit theory 2,3 and some newer theories based on quantum mechanics 4. The general principle, however, is similar to our classical mechanics’ example.
Nature has granted us miraculous machines that were synonymous with ‘life’ for billions of years. Recent works suggest, however, that natural enzymes are only a small subset of the space of enzymes that are physically and chemically possible. As we gain a deeper understanding of various mechanisms of enzyme catalysis, we expect the number of artificial enzymes catalyzing reactions not found in nature (e.g. plastic degradation) or work in conditions inhospitable for life (e.g. extreme temperatures or pH) to increase dramatically.
1. Fischer, E. Influence of configuration on the action of enzymes. Ber. Dtsch. Chem. Ges. 27, 2985–2993 (1894).
2. Pauling, L. Chemical achievement and hope for the future. Am. Sci. 36, 51–58 (1948).
3. Koshland, D. E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc. Natl. Acad. Sci. U. S. A. 44, 98–104 (1958).
4. Klinman, J. P. & Kohen, A. Hydrogen tunneling links protein dynamics to enzyme catalysis. Annu. Rev. Biochem. 82, 471–496 (2013).
